Item
アビガンで新型コロナ症状早く改善 承認を申請(2020年10月16日) - Apply for approval for early improvement of new corona symptoms with Avigan (October 16, 2020)
Title (Dublin Core)
アビガンで新型コロナ症状早く改善 承認を申請(2020年10月16日) - Apply for approval for early improvement of new corona symptoms with Avigan (October 16, 2020)
Description (Dublin Core)
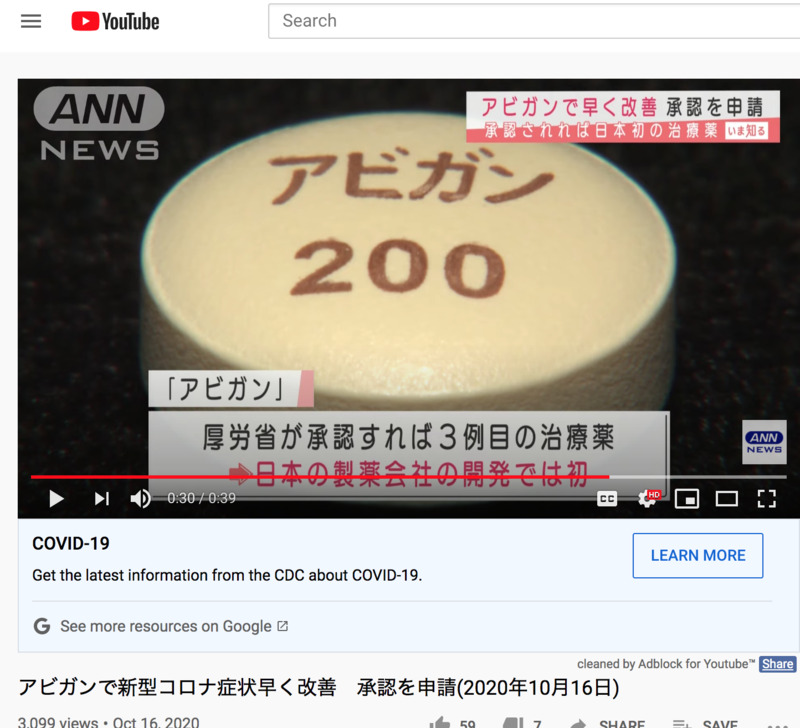
I previously uploaded a story about the drug Avigan, where FUJIFILM was still in the process of asking for approval. This news talks about how FUJIFILM now actually asked for manufacturing approval and could be another drug that could be used for the new coronavirus. Personally, I do here that this drug also has a lot of side effects for certain patients, so I feel like we are rushing the development of drug. Side note, Avigan is also known as Favipiravir which is known to be used for treating influenza mainly in Japan.
承認されれば日本の製薬会社が作る初めての新型コロナ治療薬となります。
富士フイルムは「アビガン」について、新型コロナウイルスの治療薬として製造販売の承認を申請したと発表しました。3月末から行われていた臨床試験で新型コロナの患者に対してアビガンを投与すると症状が早く改善することが確認でき、安全性の面でも新たな懸念事項は認められなかったということです。厚生労働省が承認すれば「レムデシベル」「デキサメタゾン」に続いて新型コロナの治療薬としては3例目となり、日本の製薬会社が開発した薬としては初めてとなります。
If approved, it will be the first new corona treatment made by a Japanese pharmaceutical company.
FUJIFILM announced that it has applied for manufacturing and marketing approval of "Avigan" as a therapeutic drug for the new coronavirus. In clinical trials that began at the end of March, it was confirmed that administration of Avigan to patients with the new corona improved their symptoms quickly, and there were no new safety concerns. If approved by the Ministry of Health, Labor and Welfare, it will be the third therapeutic drug for the new corona following the "Remdesiver" and "Dexamethasone", and the first drug developed by a Japanese pharmaceutical company.
承認されれば日本の製薬会社が作る初めての新型コロナ治療薬となります。
富士フイルムは「アビガン」について、新型コロナウイルスの治療薬として製造販売の承認を申請したと発表しました。3月末から行われていた臨床試験で新型コロナの患者に対してアビガンを投与すると症状が早く改善することが確認でき、安全性の面でも新たな懸念事項は認められなかったということです。厚生労働省が承認すれば「レムデシベル」「デキサメタゾン」に続いて新型コロナの治療薬としては3例目となり、日本の製薬会社が開発した薬としては初めてとなります。
If approved, it will be the first new corona treatment made by a Japanese pharmaceutical company.
FUJIFILM announced that it has applied for manufacturing and marketing approval of "Avigan" as a therapeutic drug for the new coronavirus. In clinical trials that began at the end of March, it was confirmed that administration of Avigan to patients with the new corona improved their symptoms quickly, and there were no new safety concerns. If approved by the Ministry of Health, Labor and Welfare, it will be the third therapeutic drug for the new corona following the "Remdesiver" and "Dexamethasone", and the first drug developed by a Japanese pharmaceutical company.
Date (Dublin Core)
Creator (Dublin Core)
Contributor (Dublin Core)
Event Identifier (Dublin Core)
Partner (Dublin Core)
Type (Dublin Core)
アビガンで新型コロナ症状早く改善 承認を申請(2020年10月16日) - Apply for approval for early improvement of new corona symptoms with Avigan (October 16, 2020)
Link (Bibliographic Ontology)
Controlled Vocabulary (Dublin Core)
Collection (Dublin Core)
Linked Data (Dublin Core)
Date Submitted (Dublin Core)
10/16/2020
Date Modified (Dublin Core)
10/16/2020
10/21/2020
05/17/2022
Date Created (Dublin Core)
10/16/2020
Language (Dublin Core)
Japanese
English
This item was submitted on October 16, 2020 by Youngbin Noh using the form “Share Your Story” on the site “A Journal of the Plague Year”: http://mail.covid-19archive.org/s/archive
Click here to view the collected data.